Sepsis occurs when the immune system’s response to an infection spirals out of control. This can cause organ failure and other internal damage, and even death. In U.S. hospital, sepsis is the leading cause of death.
For patients in sepsis, time to diagnosis is critical. Getting appropriate treatment right away leads to survival rates of roughly 80%. But for each hour that treatment is delayed, mortality rates increase by approximately 8%. Conventional sepsis tests can take hours or even days to generate results — far too long for optimal patient care. They are also expensive and must be run by skilled technicians in a hospital laboratory.
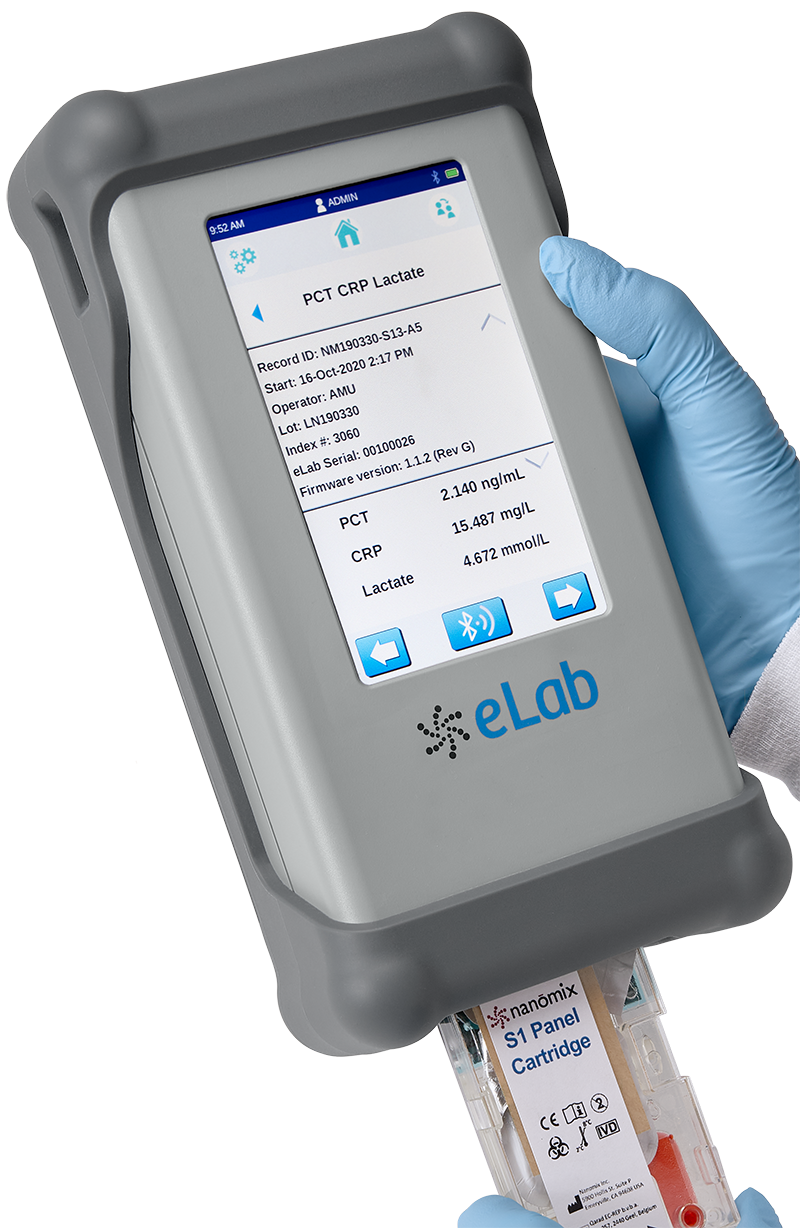
Patients in sepsis would fare better with rapid, point-of-care testing. That’s why we developed an 12-minute critical infection test to use with our handheld Nanōmix eLab®* analyzer. The test covers three key biomarkers — lactate, procalcitonin (PCT), and C-reactive protein (CRP) — to give healthcare professionals more comprehensive information to make a sepsis diagnosis. First responders and other providers can use the Nanomix test for quick, on-the-spot assessment and triage of patients no matter where they are. Information that previously took hours to get through confirmatory testing is now available to clinicians dealing with patients suffering complex and non-specific symptoms.
Key benefits of the Nanomix system for sepsis testing:
The Nanōmix critical infection test was validated through clinical trials conducted at the University of California, San Francisco; the University of California, Davis; Thomas Jefferson University; and Baylor University.